Structure-Based Design
of Bioavailable
Macrocyclic Peptide Therapeutics
Undruggable Targets | Unlimited Opportunities
Most therapeutics are either small molecule compounds or biologics. Small molecules usually act by binding to discrete hydrophobic pockets on a target protein and can be delivered into cells relatively predictably. However, the majority of therapeutic targets do not have such binding sites and so are not addressable with small molecules. Biologics, being significantly larger molecules, can disrupt biological processes in broader ways, but are largely limited to targets that are accessible from the outside of a cell. Because of this, many therapeutic targets are considered “undruggable” with current approaches. Intracellular protein-protein interactions (“PPIs”), which are central to many disease processes, including cancer metastasis, inflammation and fibrosis, are often cited as undruggable targets that could have profound therapeutic potential if tools were available to address them.
The Promise of Macrocycles
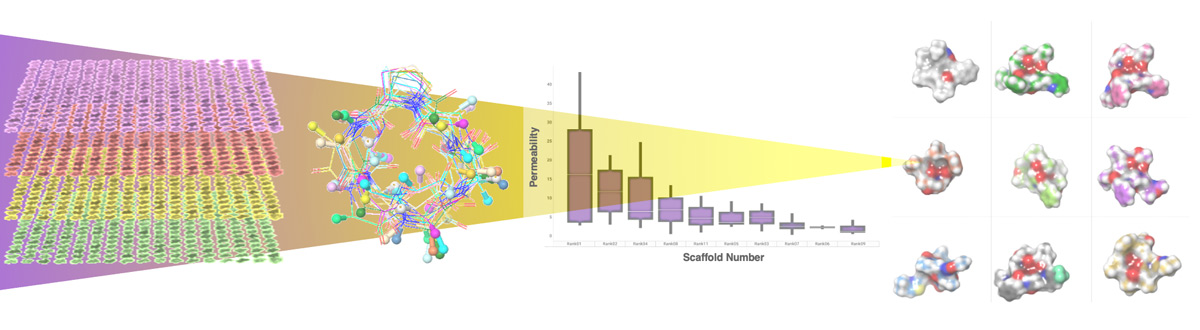
Macrocycles offer the potential to address these currently undruggable targets. They are typically comprised of cyclic peptides or peptide-like scaffold components, typically 500 to 2,000 molecular weight. Because of their size, macrocycles can interact across a relatively large area of a protein compared to small molecules. Functional side chain groups can be added to the cyclic scaffold, allowing interactions with different regions of the protein. As a result, these compounds are theoretically capable of exquisite specificity. There are a number of approved drugs in the macrocycle class, most derived from natural products. These are exemplified by cyclosporine, an orally bioavailable, passively cell permeable macrocycle that is made up of 11 amino acids (ca. 1200 molecular weight). Cyclosporine falls well outside Lipinski’s Rule of Five that predicts drug-like properties; the molecule’s conformational flexibility is believed to enable it to be cell permeable and orally bioavailable.
There have been significant efforts to develop synthetic macrocycle drugs, including large scale screening programs using natural-product derived libraries and chemistry-based approaches to constrain conformation of the molecules. Identifying macrocycles that bind with good affinity and specificity has been demonstrated to be achievable for a number of targets. However, realizing the full potential of this drug class has been challenged by difficulties in understanding and predicting pharmacokinetics, cell permeability and oral bioavailability. The conformational complexity of macrocycles has prevented, until now, a systematic approach to the design of permeable compounds in this drug class and thus has greatly limited the ability of drug developers to address important target classes, including intracellular protein-protein interactions.
In a decade-long collaboration, Circle’s scientific founders Matt Jacobson and Scott Lokey studied the properties of synthetically tractable macrocycles and developed new tools for understanding and predicting cell permeability in this drug class. Building on this foundation, Circle has developed the MXMOTM platform for the development of cell permeable macrocycle therapeutics that combines computational structure-based design with advanced, fully synthetic macrocycle chemistry. Our structure-based design approach utilizes computational modeling to design both target affinity and passive cell permeability in virtual compound libraries, which enables us to tackle important intracellular targets, including protein-protein interactions, as well as to achieve oral bioavailability. Our chemistry capabilities support the synthesis of highly diverse macrocycles, and automation enables us to assess, in parallel, multiple design hypotheses for a given target, and to rapidly explore structure activity relationships (SARs) for hit to lead progression.